Popular diabetes and weight loss drug doubles risk of eye disease that causes blindness, study warns
07/28/2025 / By Cassie B.

- Popular diabetes and weight-loss drug tirzepatide (Mounjaro/Zepbound) more than doubles the risk of severe eye disease, with 1.1% of users developing sight-threatening retinopathy compared to 0.5% of non-users.
- The study tracked 6,870 Type 2 diabetes patients, revealing a 115% higher risk of proliferative diabetic retinopathy (PDR), which can cause blindness if untreated.
- Rapid blood sugar drops from tirzepatide may trigger retinal damage, though the exact mechanism remains unclear, raising concerns about the drug’s direct effects.
- Eli Lilly, the drug’s maker, stayed silent on the findings, while another study linked GLP-1 drugs like Ozempic to doubled risks of another blinding eye condition.
- Experts urge patients on these drugs to get frequent eye screenings and watch for sudden vision changes, dark spots, or distorted lines to prevent irreversible damage.
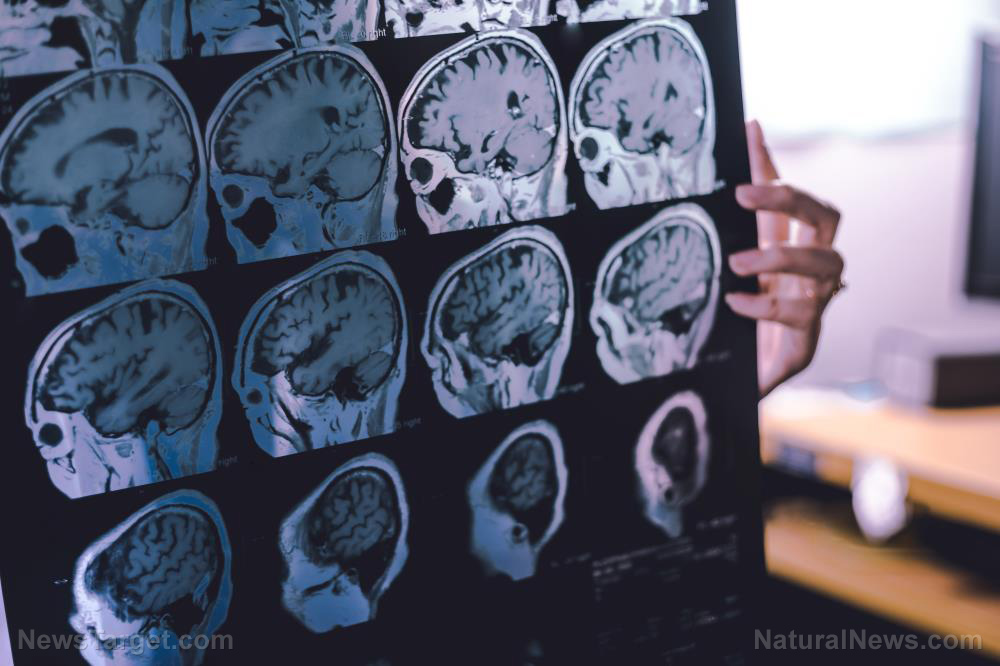
A major new study has exposed how the blockbuster diabetes and weight loss drug tirzepatide, sold under brand names Mounjaro and Zepbound, more than doubles the risk of a devastating, sight-threatening eye condition.
The research, published in Diabetologia, analyzed nearly 7,000 Type 2 diabetes patients and found that 1.1% of those taking tirzepatide developed proliferative diabetic retinopathy (PDR), compared to just 0.5% of non-users. Most victims already had mild retinal damage, with the average detection time occurring just 11 months after starting the drug.
This is yet another example of how the profit-driven medical-industrial complex pushes dangerous “miracle” drugs onto desperate patients, only for them to discover—too late—that the “cure” may be worse than the disease. And with millions now taking GLP-1 drugs like tirzepatide and semaglutide (Ozempic/Wegovy) for weight loss, often downplaying the risks, this study serves as a warning that Big Pharma’s quick fixes come with devastating consequences.
Tirzepatide users face 115% higher risk of blindness-linked condition
The real-world study, conducted by researchers at Imperial College London Diabetes Centre, tracked 6,870 adults with Type 2 diabetes, comparing 3,435 tirzepatide users to an equal number of matched non-users. The results were alarming: those on the drug faced 115% higher odds of developing PDR, the most severe stage of diabetic retinopathy.
PDR occurs when abnormal blood vessels grow on the retina, leading to vitreous hemorrhage, retinal detachment, and even blindness if untreated. While the general diabetic population has a PDR prevalence of 2.3% to 7.5%, the study found tirzepatide users had an incidence rate of seven cases per 1,000 person-years, a small but significant increase that could translate to thousands of preventable vision-loss cases as these drugs flood the market.
Sudden blood sugar drops may trigger retinal damage
Tirzepatide works by mimicking hormones that suppress appetite and stimulate insulin, leading to rapid blood sugar reductions. But this sudden drop may be the hidden culprit behind the increased PDR risk.
Dr. Meenal Agarwal, a board-certified optometrist not involved in the study, noted that rapid glycemic control has long been linked to early worsening of diabetic retinopathy. However, the study found only a modest average HbA1c drop of 0.4% among tirzepatide users, suggesting that other mechanisms—possibly the drug itself—may be accelerating retinal harm.
Big Pharma’s silence speaks volumes
Eli Lilly, the manufacturer of tirzepatide, did not respond to media requests for comment, which is a telling omission from a company raking in billions while patients gamble with their vision.
Meanwhile, another study published in JAMA Ophthalmology found that GLP-1 drugs like semaglutide (Ozempic/Wegovy) doubled the risk of neovascular age-related macular degeneration (nAMD), another blinding condition. Researchers analyzed more than 139,000 diabetic patients in Canada and discovered that those on GLP-1s for six months had twice the risk of nAMD, with the danger tripling after 30 months of use.
Dr. Rajeev H. Muni, the study’s lead investigator, cautioned: “While the absolute risk remains low, this represents a relative doubling in risk.” But when millions are taking these drugs, even a small percentage translates to countless preventable tragedies.
The urgent need for eye monitoring
The findings underscore a critical warning for diabetics and weight-loss patients: regular eye screenings are non-negotiable. Dr. Agarwal stressed that patients must watch for:
- Sudden vision changes
- Dark spots or flashes of light
- Distorted straight lines (a sign of macular swelling)
Yet how many unsuspecting users are told this before popping their first pill?
Once again, Big Pharma’s “wonder drugs” come with horrifying fine print. Tirzepatide and other GLP-1 medications may help with weight loss and blood sugar—but at what cost? Blindness? Retinal detachment?
This isn’t just a diabetes issue. With millions now taking these drugs for cosmetic weight loss, often without proper warnings, we’re staring down a public health disaster.
Sources for this article include:
Submit a correction >>
Tagged Under:
Big Pharma, diabetes, diabetic retinopathy, GLP-1 drugs, medical violence, pharma fraud, prescription warning, semaglutide, tirzepatide
This article may contain statements that reflect the opinion of the author