Advertising LIES: Researchers discover that most moisturizers on the market are dishonest in their advertising
10/05/2017 / By Russel Davis
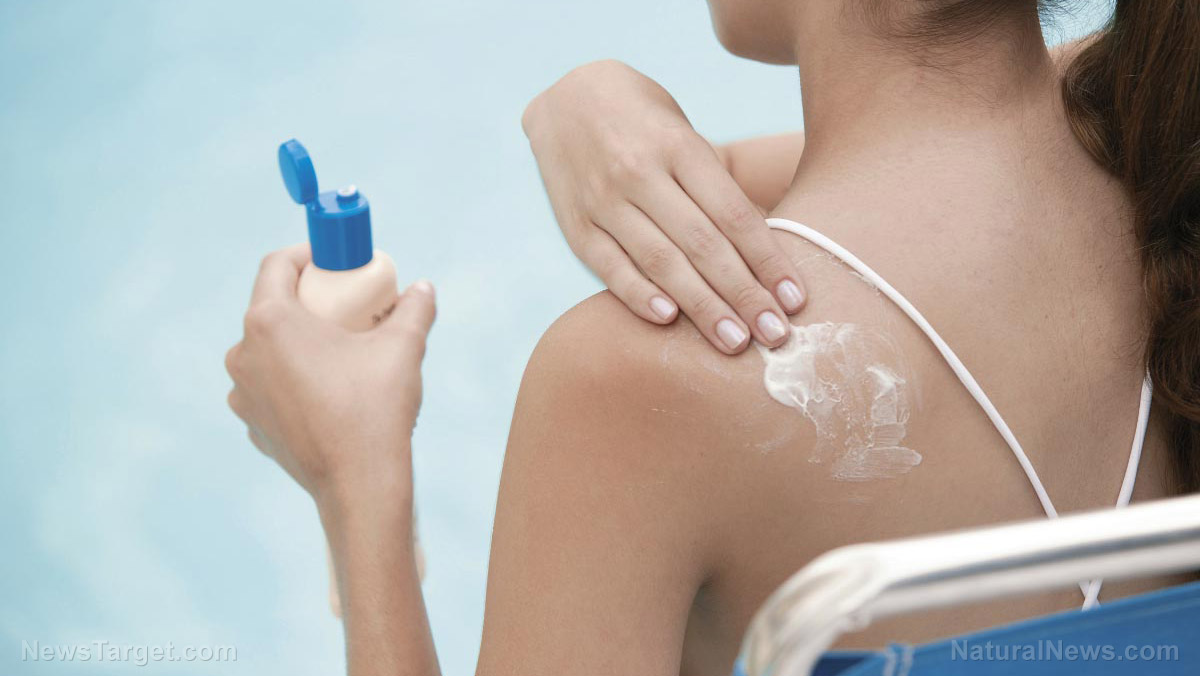
A recent study in JAMA Dermatology has revealed that some of the most popular brands of moisturizers may contain allergens and perfume despite claims of being hypoallergenic and fragrance-free. According to researchers at the Northwestern University in Chicago, moisturizing creams do not require approval from the United States Food and Drugs Administration as the federal agency considers such products as cosmetics. This further puts the users at risk of adverse skin conditions, the researchers have added.
The research team has examined 174 top-selling skin moisturizers and their ingredients to check if the manufacturers have been honest about the content displayed in the product labels. The health experts have found that 83 percent of products labeled hypoallergenic actually contained allergens that may trigger severe allergic reactions and skin disorders. Likewise, the researchers have observed that 45 percent of the moisturizers labeled as fragrance-free did contain perfume.
“The cosmetics industry is highly competitive and if someone can easily copy someone else’s successful cosmetic, that would be a competitive disadvantage…I don’t think it’s too much to ask of manufacturers that they [be required to] register what they’re selling so that it can be tracked…What we mean is, using the least amount of products with the least amount of potentially allergenic materials or chemicals in them to reduce the risk,” former FDA commissioner Dr. Robert Califf has told the National Public Radio online.
False advertising seen in popular moisturizing creams
The research team has found that the Gold Bond Ultimate Healing Skin Lotion with Aloe contained up to six allergenic ingredients in the mixture, making it one of the highest allergen-containing among the examined products. St. Ives Advanced Therapy Body Lotion also contained as much as five allergenic ingredients, while Suave Smoothing Cream and NIVEA Smooth Daily Moisture Body Lotion each had four allergens in their mixtures. (Related: Skin care expert reveals the 20 most toxic chemical ingredients in beauty products… are you poisoning yourself?)

In addition, Neutrogena Oil-Free Moisture SPF 35 is found to contain up to three allergenic ingredients in its mixture. The same number of allergens has been seen in Cetaphil Restoraderm Eczema Calming Body Moisturizer and Palmer’s Coco Butter Formula with Vitamin E. The research team has also observed that CeraVe Moisturizing Cream and Burt’s Bees Mama Bee Belly Butter each contained two allergenic components.
Dr. Steve Xu, a dermatologist at Northwestern’s Feinberg School of Medicine and the study’s lead researcher, stressed that consumers should exercise caution in purchasing products that claim to be dermatologist-recommended. According to Dr. Xu, the products could have been tested by a varying number of professionals, from as few as three to as many as a thousand. This affects the accuracy of the skin products, Dr. Xu added.
The research team has also suggested various ways on how the FDA could better regulate the skin care products.
“First, Congress should provide the agency with an adequate budget to fulfill its existing responsibilities, which it mandates. The FDA is vastly underresourced for even the very limited responsibilities it currently has for the safety of cosmetics. Second, Congress should require manufacturers to register marketed products. The FDA has a voluntary registration system for cosmetics. Without mandatory registration, however, the agency has no way to determine the universe of products to which consumers are exposed.
Absent such data, the FDA’s task of discerning important safety signals with passive surveillance alone is prohibitively difficult. Finally, self-regulation of cosmetics is the current default policy. By borrowing innovations being developed for efficient, cost-effective active surveillance of drugs and devices, the oversight of cosmetics can be modernized without creating an inappropriately burdensome regulatory process,” the researchers have suggested.
The FDA currently considers moisturizers as cosmetic products, and as such do not require pre-approval to enter the market. However, the federal agency has the power to take the product off the market if claims of adverse skin effects have been proven true.
Sources include:
Submit a correction >>
Tagged Under:
Cosmetics, dishonest, fragrance, ingredients, moisturizers, perfumes, product labels, Skin, skin care, skin care products
This article may contain statements that reflect the opinion of the author